A range of approaches are applied to the dissection of enzyme mechanisms and the role of enzyme-substrate binding energy in catalysis. These include steady and pre-steady state kinetic analyses with wild type and mutant enzymes. Specific approaches employed include measurement of linear free energy relationships with a range of synthetic substrates, kinetic isotope effect analysis and enzyme inhibitor design, synthesis and evaluation.
Mechanism-based labelling studies, in conjunction with liquid chromatography coupled to mass spectrometry, are used to identify key active site residues. A particular emphasis has been placed upon the development of methods to trap and probe reaction intermediates in enzymatic catalysis using mechanism-based inhibitors. These inhibitors, as well as tightly bound transition state analogues, are employed in structural studies to probe active site structure and substrate recognition and distortion. Collaborative NMR studies are also used to probe issues of bound substrate conformation and ionisation state as well as probing electrostatic interactions and dynamics within the enzyme active site along the reaction coordinate.
Classical Mechanisms of Glycosidases – Probing Fine Details
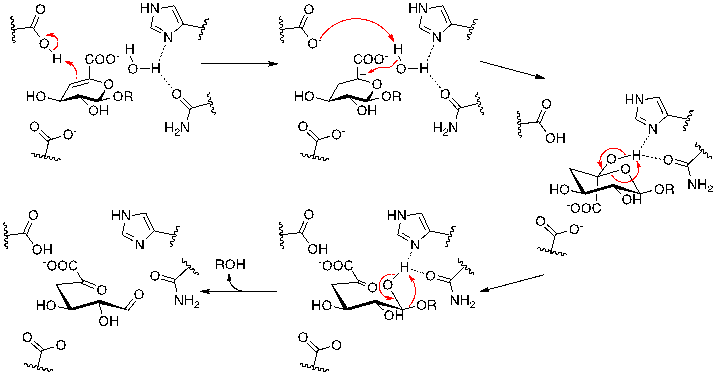
The two canonical mechanisms of glycosidases cleaving with inversion or retention of anomeric configuration were proposed by Koshland and have been shown by this group and others to involve two key active site carboxylic acids, as shown by the following inverting and retaining mechanisms.
Current efforts to further understand how these enzymes accelerate these reactions by factors of >1017 fold focus upon the roles of substrate distortion, the active site electrostatic environment and its modulation during catalysis, the role of individual binding interactions in transition state stabilization and the role of motion in catalysis. Of particular importance have been our detailed studies on the two xylanases Cex (33 kDa) and Bcx (20 kDa) which appear to carry out hydrolysis via quite different transition states. (Jacqueline Wicki, David Poon, Terrence Raasch, Ian Greig, Luke Lairson, Miriam Kötzler, Hongming Chen)
Variations on a Theme: Alternative Glycoside Cleavage Mechanisms
We have been uncovering variations on these mechanisms and continue to probe them. Examples include:
Family GH88 unsaturated uronic acid hydrolases: these enzymes remove the unsaturated sugar left at the non-reducing terminus by polysaccharide lyases using a hydration mechanism.
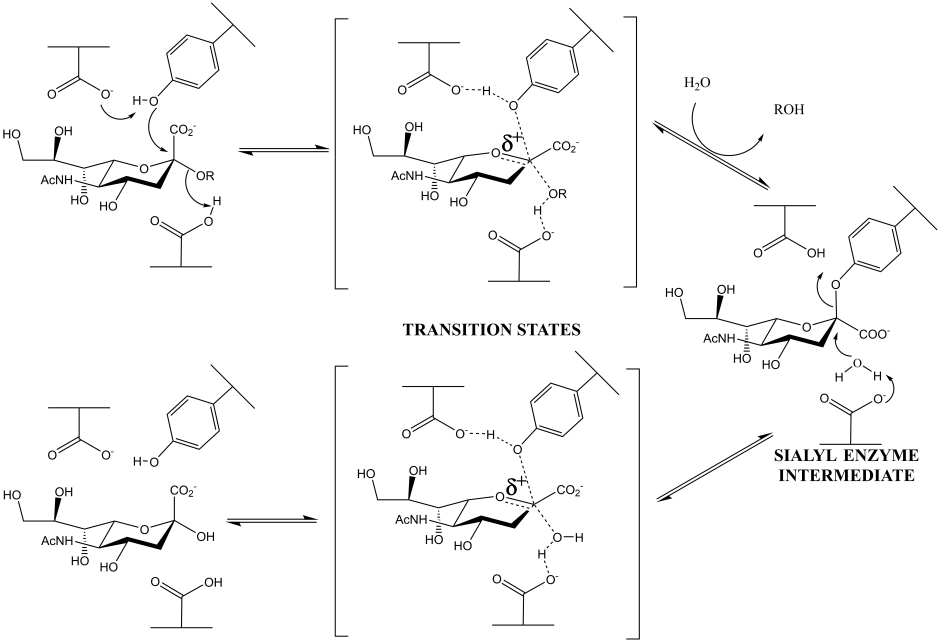
Family GH33 & 34 sialidases and trans-sialidases: these enzymes, which cleave a substrate containing a carboxylic acid at its anionic center, have now been shown to use tyrosine as the catalytic nucleophile. (Andrew Watts, Iben Damager, Mark Chen, Yuan Yao, Jin-Hyo Kim, Tom Wennekes, Sabrina Buchini, Ricardo Resende, Stefan Lutz, Zhizeng Gao, Kyle Robinson)
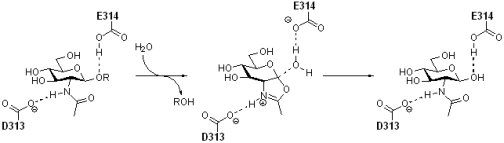
Family of GH18 & 20 N-acetyl hexosaminidases: many of these enzymes employ the substrate’s own N-acetyl group as the catalytic nucleophile with reaction proceeding via an oxazolinium intermediate. (Ian Grieg, James MacDonald, Dave Vocadlo, Berit Aam, Andrés Gonzalez-Santana)
Family GH31 α-glucan lyases: these enzymes cleave glycosides by elimination through the two-step mechanism shown below. (Seung-Seo Lee, Young-Wan Kim, Ran Zhang)
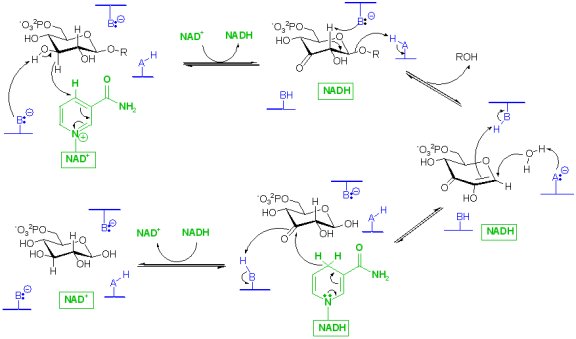
Family GH4 glucosidases: a redox mechamism. This unusual enzyme class employs a tightly bound NAD to facilitate glycoside cleavage via an oxidation, elimination, addition, reduction sequence. (Vivian Yip, Yunsong Li)
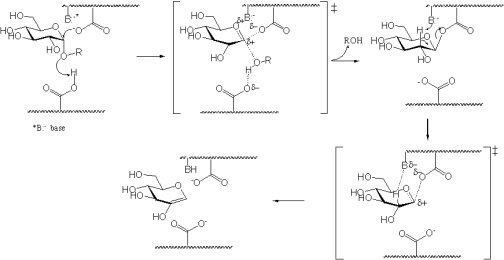
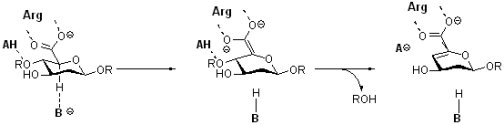
Family PL4 polysaccharide lyases: these enzymes degrade polysaccharides containing uronic acids (sugars with a carboxylic acid at C6) via an E1cb elimination mechanism. (Carl Rye, James MacDonald)
Family GH88 unsaturated uronic acid hydrolases: these enzymes remove the unsaturated sugar left at the non-reducing terminus by polysaccharide lyases using a hydration mechanism.
Glycosyl transferases: the enzymes that synthesize glycosides, typically using a nucleotide phophosugar such as UDP glucose as a glycosyl donor. Mechanisms of inverting glycosyl transferases appear to be simple analogues of the inverting glycosidases. Our studies in this class are represented by the sialyl transferase of Campylobacter. Mechanisms of retaining glycosyl transferases, however, are far from clear. Our efforts have focused heavily upon the α-galactosyl transferase IgtC from Neisseria. After many efforts to trap possible glycosyl enzyme intermediates we and others have settled on an unusual SNi mechanism as the most probable. All such studies are in collaboration with Warren Wakarchuk (Ryerson University, Toronto), Natalie Strynadka and Lawrence McIntosh (UBC) and are funded by the Canadian Institutes for Health Research (CIHR). (Luke Lairson, Chris Tarling, Hao Ly, Fathima Shaikh, Amir Aharoni, Patrick Chan, Francesco Rao, Bojana Rakic, Ching-Ching Yu, Lars Baumann, David Kwan, Kevin Mehr)